
Compressed air is in a key component in many pharmaceutical manufacturing operations. Air power is used in processes such as tablet manufacturing, cleaning and drying, transporting products, packaging and more.[1]
Because compressed air has such close proximity to products, extremely high air purity is required. Increasingly stringent regulations on purity have many pharmaceutical manufacturing operations using Clean Room Classifications as outlined by ISO 14644.
A clean room is classified by the maximum acceptable number of parts, by size, in the air per cubic meter. Clean rooms must meet stringent regulatory requirements and must be monitored regularly for compliance.
This often means clean rooms require more energy, air, and advanced technology to maintain the required conditions.[2]
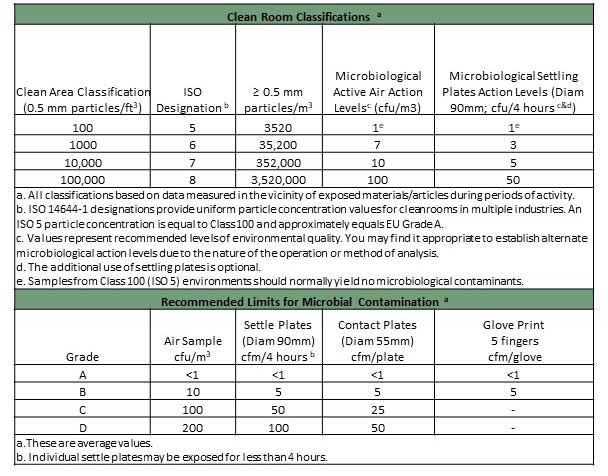
Chart source: https://www.airchecklab.com/resources/standards-specifications/microbial-cleanroom-compressed-air-classifications/
Air purity requirements depend on the application and the specific requirements of the clean room. The International Society for Pharmaceutical Engineers (ISPE) Good Practice Guide specifies, in cases where the gas is entering a classified area, it is required to at least meet the room classification limits established for the cleanroom environment
(2016).
The recent US FDA Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice also recommends compressed gas should be of appropriate purity… and it’s microbiological and particle quality after filtration should be equal to or better than that of the air in the environment into which the gas is introduced.
Air compressors pull in ambient air. The purity of this air is highly impacted by environmental factors. For example, if the compressor is pulling ambient air from near a construction site, the particulates from the job site can be pulled into the compressor and contaminate the process air if the proper filtration and purity procedures are not followed.
This can pose a huge risk to the quality of products and the safety of the consumers. In addition, it can reduce the efficiency of the compressed air system and put additional wear and tear on each component.[3] Increasing energy costs, repair costs and the likelihood of unplanned downtime.
That is why it is critical to not only have the appropriate system in place, but to monitor that system at all times to help ensure all purity requirements are being met.
Sullair offers a broad line of oil free air compressors, air treatment products and compressor monitoring technology to meet your needs. And, the Sullair network of Authorized Distributors are true compressor experts ready to help you build a reliable and efficient system to help keep your operation running smoothly for years to come.
Use the checkboxes to select the types of cookies you want to accept, then press the “Save Settings” button. View our Privacy Policy.